Is chemistry just chemistry? Absolutely not. The realm of chemical sciences is far more diverse than many realize, with distinct disciplines demanding unique skill sets and offering specialized career paths. When students first encounter the term "organic chemistry," a common question arises: Isn't all chemistry fundamentally the same? The answer, while seemingly simple, unfolds into a complex and fascinating exploration of the building blocks of life and the reactions that govern their behavior.
While both general chemistry and organic chemistry reside under the expansive umbrella of chemical sciences, their specific focuses, methodological approaches, and inherent challenges set them apart. Understanding these key distinctions is vital for students to adequately prepare for the academic rigor and to ultimately excel in both domains. General chemistry provides the foundational principles, introducing concepts like atomic structure, bonding, stoichiometry, and thermodynamics. Organic chemistry, on the other hand, delves into the intricate world of carbon-containing compounds, exploring their structures, properties, reactions, and applications. Mastering organic chemistry often involves navigating complex synthesis pathways and unraveling intricate reaction mechanisms, a process many find profoundly challenging.
| Field | Description | Typical Topics | Applications |
|---|---|---|---|
| General Chemistry | The foundation of all chemistry, covering fundamental principles and laws. | Atomic structure, bonding, stoichiometry, thermodynamics, kinetics, acids and bases, equilibrium. | Serves as a prerequisite for many science and engineering fields; essential for understanding basic chemical processes. |
| Organic Chemistry | The study of carbon-containing compounds, their structures, properties, reactions, and synthesis. | Alkanes, alkenes, alkynes, alcohols, ethers, aldehydes, ketones, carboxylic acids, amines, spectroscopy, reaction mechanisms. | Pharmaceuticals, plastics, polymers, agriculture, biochemistry, materials science. |
| Inorganic Chemistry | The study of compounds that are not primarily carbon-based, including metals, minerals, and catalysts. | Coordination chemistry, solid-state chemistry, organometallic chemistry, catalysis, materials science. | Catalysis, materials science, electronics, pigments, medicine. |
| Analytical Chemistry | The study of the composition of matter, both qualitative and quantitative. | Spectroscopy, chromatography, electrochemistry, titrations, statistical analysis. | Environmental monitoring, quality control, forensics, food safety. |
| Physical Chemistry | The study of the physical principles underlying chemical phenomena. | Thermodynamics, kinetics, quantum mechanics, statistical mechanics, spectroscopy. | Materials science, chemical engineering, biochemistry. |
| Biochemistry | The study of the chemical processes within living organisms. | Proteins, carbohydrates, lipids, nucleic acids, enzymes, metabolism. | Medicine, biotechnology, agriculture. |
Reference: American Chemical Society (ACS)
- Dee From Clueless The Ultimate Dionne Davenport Guide
- Jay Harringtons Wife Truth About Divorce New Love Life
Many students find the transition from general chemistry to organic chemistry a significant leap. Organic chemistry demands a robust understanding of general chemistry principles, coupled with advanced mathematical skills. This requirement alone can present a substantial obstacle for many. The sheer volume of information in organic chemistry, combined with the necessity of applying complex rules and theories to solve problems, can feel overwhelming, resulting in a steep and often daunting learning curve. This is why many institutions offer organic chemistry as a core academic requirement, ensuring students develop critical thinking and problem-solving skills applicable across various disciplines.
The curriculum of a typical university-level general chemistry course usually spans two semesters and encompasses a broad range of topics. These topics often include the periodic table of elements, a fundamental tool for organizing and understanding chemical properties. Students learn about atomic structure, electron configurations, and the trends in electronegativity, ionization energy, and atomic size. Chemical bonding, including ionic, covalent, and metallic bonds, is another crucial area of study, allowing students to predict the properties of molecules and compounds. Stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions, is also covered extensively, enabling students to balance chemical equations and calculate yields. Furthermore, general chemistry delves into the principles of thermodynamics, exploring concepts like enthalpy, entropy, and Gibbs free energy to understand the spontaneity and equilibrium of chemical reactions.
Acid-base chemistry is another critical component of general chemistry, with students learning about pH, titration, and buffer solutions. Oxidation-reduction reactions, also known as redox reactions, are explored, focusing on the transfer of electrons and the concepts of oxidation states. Kinetics, the study of reaction rates and mechanisms, provides insights into how reactions proceed and the factors that influence their speed. Equilibrium, both chemical and physical, is also a major topic, allowing students to predict the direction a reaction will shift under varying conditions. Finally, many general chemistry courses include an introduction to nuclear chemistry, covering radioactivity, nuclear reactions, and their applications.
- Gorr The God Butcher Comics Vs Mcu Explained Powers Guide
- Olivier Rousteings Balmain The Future Of Fashion
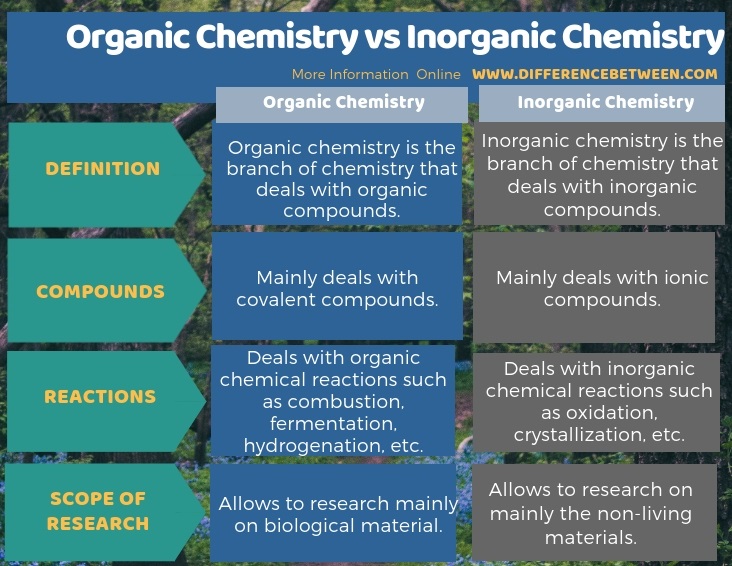
In stark contrast, organic chemistry focuses almost exclusively on the study of carbon compounds. While general chemistry introduces the basic principles applicable to all chemical substances, organic chemistry hones in on the unique bonding properties of carbon, the vast diversity of carbon-based molecules, and their reactivity. The study of inorganic compounds, generally defined as those without carbon, falls outside the realm of organic chemistry. This division allows for a more in-depth examination of the complexities and nuances associated with carbon chemistry.
The core of organic chemistry revolves around understanding the structure, nomenclature, properties, and reactions of various classes of organic compounds. Alkanes, alkenes, alkynes, alcohols, ethers, aldehydes, ketones, carboxylic acids, amines, and amides are all extensively studied. Students learn how to name these compounds using IUPAC nomenclature, predict their physical and chemical properties based on their structure, and understand the mechanisms by which they react with other molecules. Spectroscopic techniques, such as NMR, IR, and mass spectrometry, are also integral to organic chemistry, allowing students to identify and characterize unknown organic compounds. This skill is essential for researchers and professionals working in various fields, including pharmaceuticals, materials science, and environmental chemistry.
Reaction mechanisms are a cornerstone of organic chemistry. Understanding how reactions occur at a molecular level is crucial for predicting the products of reactions, designing new synthetic routes, and controlling reaction outcomes. Students learn about various types of reaction mechanisms, including SN1, SN2, E1, and E2 reactions, as well as addition, elimination, substitution, and rearrangement reactions. By understanding the factors that influence these mechanisms, students can design and optimize synthetic strategies for creating complex organic molecules. This focus on reaction mechanisms is what truly sets organic chemistry apart from general chemistry.
The later stages of an organic chemistry course often bridge the gap with biochemistry, exploring the chemistry of biological molecules. Carbohydrates, lipids, amino acids, proteins, and nucleic acids, the building blocks of life, are examined in detail. Students learn about their structures, properties, and roles in biological systems. This connection to biochemistry highlights the importance of organic chemistry in understanding life processes and developing new medical treatments. Organic chemistry plays a pivotal role in the synthesis of pharmaceuticals, including antibiotics, antivirals, and anticancer drugs. It also underpins the development of new materials, such as polymers and plastics, which have revolutionized modern life. In essence, organic chemistry is central to many aspects of our daily lives, from the food we eat to the clothes we wear.
The choice between focusing on general chemistry versus organic chemistry can significantly influence not only your comprehension of fundamental chemical principles but also your future career prospects. A solid understanding of general chemistry is often a prerequisite for many STEM fields, providing the groundwork for further studies in areas such as engineering, medicine, and environmental science. On the other hand, a specialization in organic chemistry opens doors to careers in pharmaceuticals, materials science, and biotechnology, where the ability to synthesize and characterize complex organic molecules is highly valued.
Choosing the right path depends on your interests and career goals. If you are passionate about understanding the fundamental principles of chemistry and how they apply to a broad range of phenomena, general chemistry might be the more suitable choice. However, if you are fascinated by the intricate world of carbon compounds and their role in life, health, and technology, then organic chemistry is likely the path for you. Ultimately, both general chemistry and organic chemistry offer rewarding career opportunities and contribute significantly to our understanding of the world around us.
To excel in organic chemistry, several strategies can be employed. First and foremost, it's crucial to build a strong foundation in general chemistry. Understanding the basic principles of bonding, stoichiometry, and thermodynamics will make it easier to grasp the more complex concepts in organic chemistry. Second, practice is essential. Work through as many problems as possible, focusing on understanding the underlying concepts rather than memorizing facts. Use textbooks like Kline and McMurrys to solidify your understanding. Third, don't be afraid to ask for help. Attend office hours, join study groups, and seek out tutoring if needed. Finally, stay organized and manage your time effectively. Organic chemistry requires consistent effort and dedication, so it's important to stay on top of the material and avoid cramming.
Various resources are available to help students succeed in organic chemistry. Online video lectures, such as those offered by Chad, can provide a comprehensive and engaging introduction to the subject. Practice quizzes and exams can help assess your understanding and identify areas where you need more work. Flashcards are a useful tool for memorizing important facts and reactions. Textbooks, such as "The Basics of General, Organic, and Biological Chemistry" by David W., provide a comprehensive overview of the subject matter. Utilizing a combination of these resources can significantly improve your chances of success in organic chemistry.
Moreover, understanding the reagents used in organic chemistry reactions is paramount. The organic chemistry reagent guide summarizes reactions from the perspective of the reagent used in a given transformation. For example, lithium aluminum hydride (LiAlH4) is used for the reduction of aldehydes and ketones, but it is also used for converting esters and other carbonyl derivatives into alcohols. Recognizing the specific function of each reagent is key to predicting the outcome of reactions and designing synthetic strategies. This comprehensive knowledge of reagents, combined with a strong understanding of reaction mechanisms, will enable you to tackle complex synthetic problems with confidence. Nomenclature of organic compounds and functional groups is also critical.
The applications of organic chemistry are vast and diverse. It plays a crucial role in the development of new drugs, the creation of novel materials, and the understanding of biological processes. Discoveries in organic chemistry are often made by professionals from various fields, including physicists, biologists, chemical engineers, and pharmacists, highlighting its interdisciplinary nature. From designing new polymers for high-performance plastics to synthesizing complex natural products with medicinal properties, organic chemistry has a profound impact on our world. Its ability to synthesize different compounds, including drugs that can correct biochemical anomalies, makes it an indispensable field in modern science.
In conclusion, while general chemistry and organic chemistry are both essential branches of chemical science, they differ significantly in their focus and approach. General chemistry provides the foundational principles, while organic chemistry delves into the intricate world of carbon compounds. Understanding these differences is crucial for students to succeed in their studies and pursue rewarding careers in science and technology. By building a strong foundation in general chemistry, mastering reaction mechanisms, and utilizing available resources, students can overcome the challenges of organic chemistry and unlock its vast potential. Essentially, organic compounds are almost ubiquitous, and nearly all the studies undertaken in biochemistry involve these compounds, underscoring the vital role organic chemistry plays in understanding life itself.
- Kylie Jenners Family Siblings Facts Relationships Explained
- Hey There Delilah Easy Guitar Tabs Chords Tips Learn Now
![Types of Organic Chemistry Formulae [Infographic] Chemistry.Com.Pk](https://i2.wp.com/chemistry.com.pk/wp-content/uploads/2014/08/Types-of-Organic-Formulae.png)